My first long-term research project (and undergraduate thesis!) was determining if there was any difference between the structure of ice that contained ammonia and pure water ice. This research was inspired by icy satellites throughout the solar system such as Charon and Enceladus that have been found to contain ammonia and ice. Typically, ice forms a thick crustal layer on icy satellites that contain compounds such as ammonia. Icy crustal formations on planetary satellites are affected by a variety of stresses, including tidal pull from neighboring planets and other satellites and vertical temperature differentials from deep within the crust to the surface.
The material below is all from my undergraduate thesis, with significant edits to condense the material and for ease of reading. I really enjoyed this research topic, even if it meant I spent most of my time in a very cold freezer!
why ammonia + ice?
In my research, I was trying to determine the dihedral angle of ammonia + ice. The plus sign here is intentional, as the system contains both, with no real mixing like if they were both liquid. First, on why this is important:
The dihedral angle in this type of ice will be used to infer rheological characteristics and eventually will help predict crustal behavior on icy satellites. The rheology of a material is the behavior it displays when placed under stress, and in partially molten systems like icy crusts, rheological characteristics are determined by the equilibrium microstructure between the solid and melt, defined as the dihedral angle (this is a pretty good primer on ice rheology). Dihedral angles of crystalline solids determine a variety of physical characteristics for the material and the rheological characteristics of ice are closely related to this microstructure.
Of additional interest is that having a large wetted area at the intersection between ice crystals may provide a path for materials from the inside of the icy crust to get to the surface, which could mean that surface samples (from something like the Curiosity rover) can indicate what is happening beneath the surface!
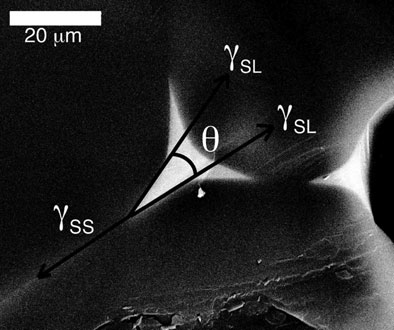
The dihedral angle between these ice crystals is marked here as theta.
what is the dihedral angle?
The dihedral angle is the angle of the solid-liquid interface towards the opposite solid solid interfaces with a vertex at the solid-solid interface, indicated by theta in the picture on the right. Since the freezing temperature of water is higher than that of ammonia, pockets of ammonia liquid solution with increasing concentration are squeezed out of the water ice crystalline solid as it freezes, when the temperature decreases. Due to the increase in ammonia concentration in the remaining liquid as the pure water crystals freeze, the liquid needs to be significantly colder to freeze than pure water. This phenomena follows the liquidus curve in the eutectic phase diagram, which gives an idea of how concentrated the ammonia will be at a particular temperature.
On an icy satellite, this means that the icy crust will have little pockets of liquid ammonia between the ice crystals, which affects how the ice behaves when, for example, a planet or other satellite causes a tidal pull on a sub-surface ocean.
how were samples made?
Samples were made by mixing bulk ice (which was painstakingly made in advance to be pure) and an ammonia solution and freezing at -17 ˚C (equivalent to 1 ˚F) . Samples were held at that temperature for up to three weeks to allow them to reach equilibrium structure at that temperature and then quenched in liquid nitrogen (-196 ˚C / -321 ˚F) to completely freeze in the ice + liquid structure. Samples were then prepared for observation, which meant quickly getting them shaved down to eliminate any odd surface structures and under a microscope to be photographed. Later, the dihedral angle was measured using software that could measure angles on the images. Over the course of a few months, hundreds of pictures led to thousands of measurements!
pictures!
Dihedral angle size and communication between ice crystal junctions. The line down the center of the ice crystals is from the blade used to shave the sample.
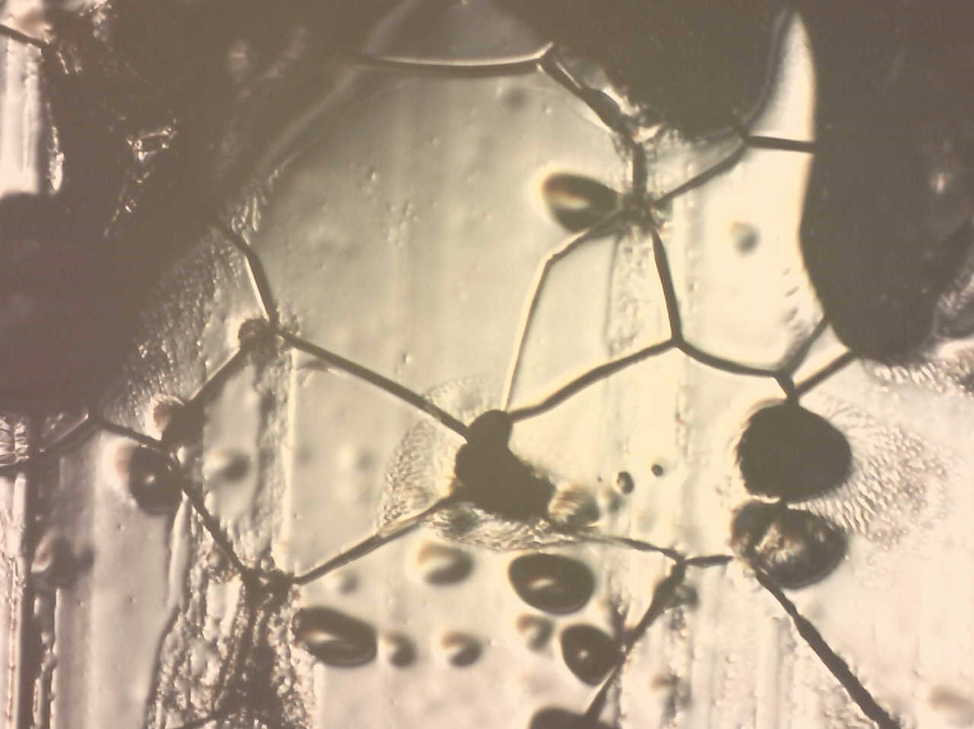
Two sets of crystal intersections. In the lower middle-left of the figure, a dry intersection is observed. In the upper middle, a wetted intersection is observed.
A field of ice crystals with a lot of different triple intersections.